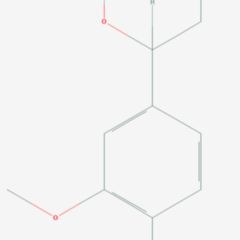
3,4-Dimethoxyphenethylamine (DMPEA), also known as O,O-dimethyldopamine, is a chemical compound of the phenethylamine class. It is an analogue of the major human neurotransmitter dopamine where the 3- and 4-position hydroxy groups have been replaced with methoxy groups. It is also closely related to mescaline which is 3,4,5-trimethoxyphenethylamine and to 3,4-dimethoxyamphetamine (3,4-DMA).
Pharmacology
DMPEA shows weak affinity for serotonin receptors. It induces the head-twitch response, a behavioral proxy of serotonergic psychedelic effects, in rodents. However, according to Alexander Shulgin, it was inactive in humans at doses of 1,000 mg orally and 10 mg intravenously.
DMPEA has some activity as a monoamine oxidase inhibitor.
Chemistry
One of the earliest syntheses of DMPEA (then referred to as "homoveratrylamine") was that of Pictet and Finkelstein, who made it in a multi-step sequence starting from vanillin. A similar sequence was subsequently reported by Buck and Perkin, as follows:
- 3,4-Dimethoxybenzaldehyde (veratraldehyde) → 3,4-Dimethoxycinnamic acid → 3,4-Dimethoxyphenylpropionic acid → 3,4-Dimethoxyphenylpropionamide → 3,4-Dimethoxyphenethylamine
A much shorter synthesis is given by Shulgin and Shulgin:
Derivatives
A known use was in the synthesis of bevantolol.
Natural occurrence
DMPEA occurs naturally along with mescaline in various species of cacti such as San Pedro and Peruvian Torch.
See also
- 3-Methoxytyramine
- Mescaline
References
External links
- DMPEA Entry in PiHKAL
- DMPEA Entry in PiHKAL • info